Latest News
Global Leader
in Stem Cell Therapeutics
0+
Number of registered patents
in Korea and overseas
0+
Number of national R&D
grants received in Korea
0
Number of private cord blood
units stored
0%
Percentage of
R&D staff
First-Generation Stem Cell Products
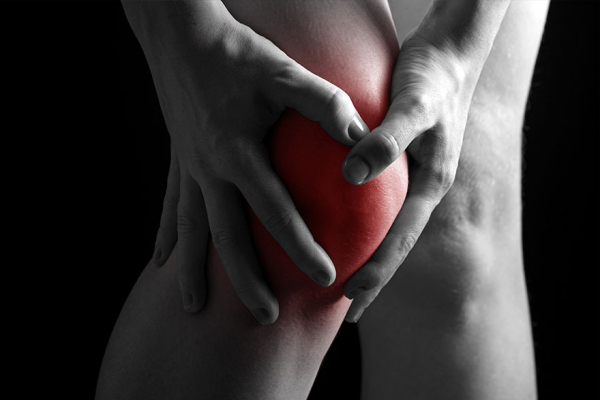
CARTISTEM®
Treatment of knee cartilage defects including for patients with Knee Osteoarthritis
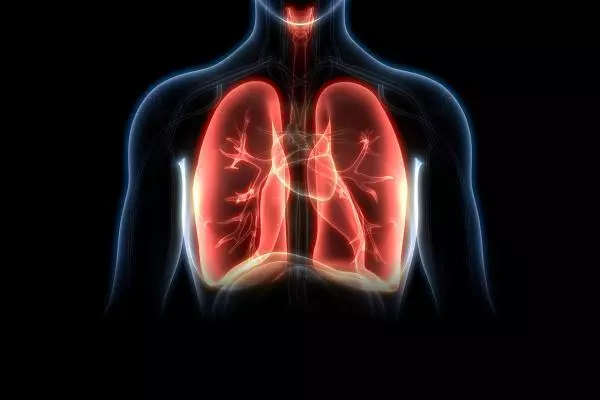
PNEUMOSTEM®
Preventive treatment of bronchopulmonary dysplasia(BPD)
Second-Generation Stem Cell Products
SMUP-IA-01
Intra-articular Injectable treatment for osteoarthritis
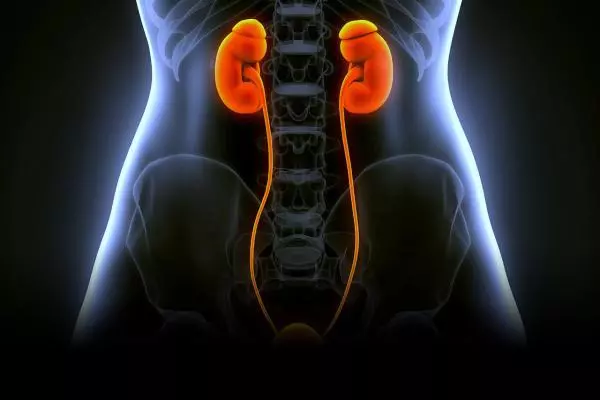
SMUP-IV-01
Intra-venous infusion treatment for diabetic nephropathy
Global Leader in Stem Cell Therapeutics
As a global leader in biotechnology with cutting-edge Technologies in Stem Cell Therapeutics, MEDIPOST continues to innovate and challenge to tackle unmet medical needs.