Preventive treatment of bronchopulmonary dysplasia(BPD)
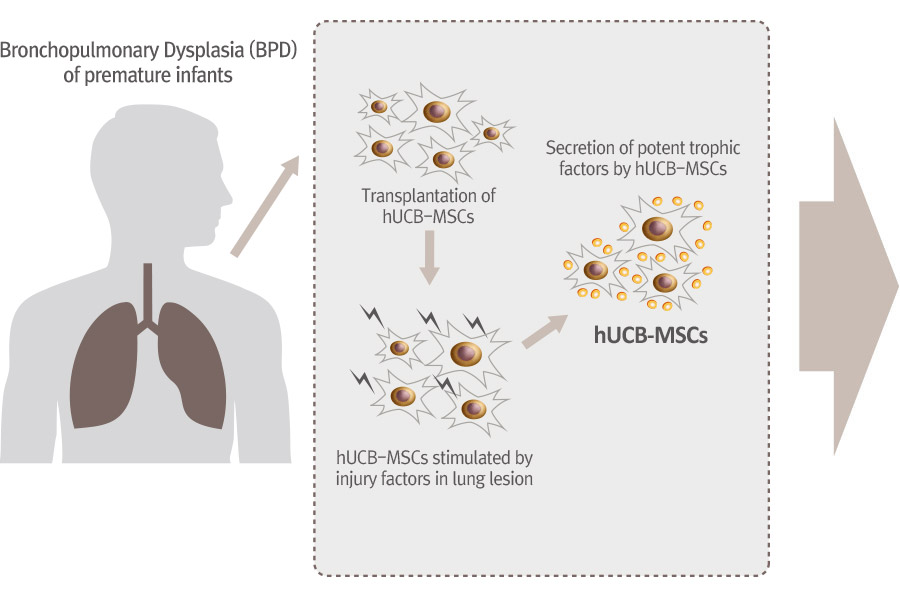
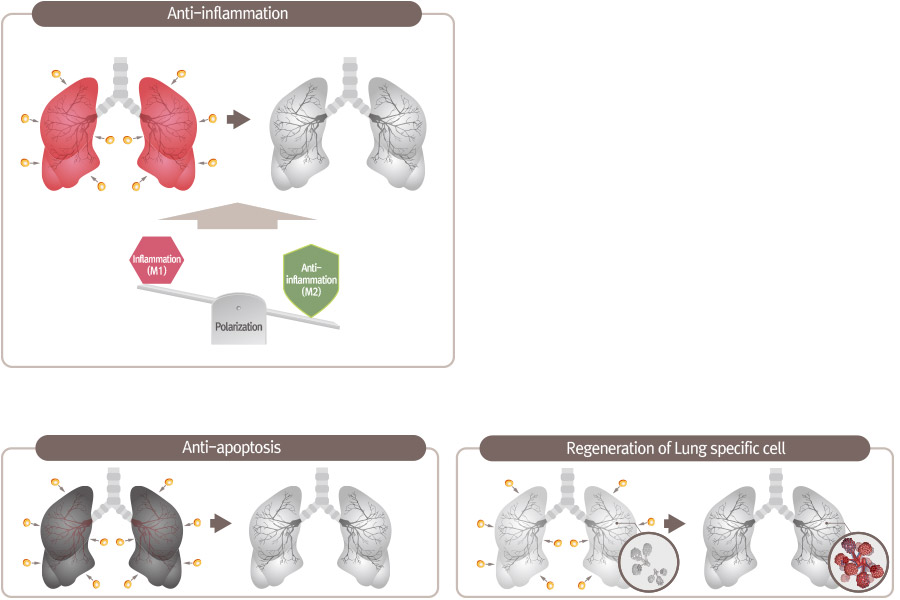
PNEUMOSTEM® an allogeneic umbilical cord blood-derived mesenchymal stem cell product, is currently under development for the preventive treatment of Bronchopulmonary Dysplasia(BPD) in premature infants. Currently, Phase 2 clinical trial is ongoing in Korea and PNEUMOSTEM® has been Orphan Drug designated by US FDA and EMA. Recently, US FDA granted a Fast Track Designation for PNEUMOSTEM®.
| · Product : | PNEUMOSTEM® (human Umbilical Cord Blood-derived Mesenchymal Stem Cells) |
|---|---|
| · Manufacturer : | MEDIPOST Co., Ltd. |
| · Indication : | Preventive treatment of Bronchopulmonary Dysplasia(BPD) |
| · Route of Administration : | Intra-tracheal administration |
Mechanism of Action for PNEUMOSTEM®