MEDIPOST disclosed on 27 March that the accumulative number of patients administered with cartilage regerative stem cell treatment ‘CARTISTEM®‘ has risen above 10 million.
‘CARTISTEM®’ had gradually increasing prescription every month after its first patient administration in April 2012, and recorded 1,000 patients after 1 year and 11 months. The number of vials administered to patients is 1,000 over 1,100 vials.
This number excludes donations and free supplies, but includes administration surgeries overseas including Hong Kong.
MEDIPOST explained htat ‘CARTISTEM®’ is a latest technology medicine different to existing medicinal products, is a prescription medicine for which marketing is not permitted, and considering that stem cell treatments have not been popularized, 1,000 patients is a record with great meaning.
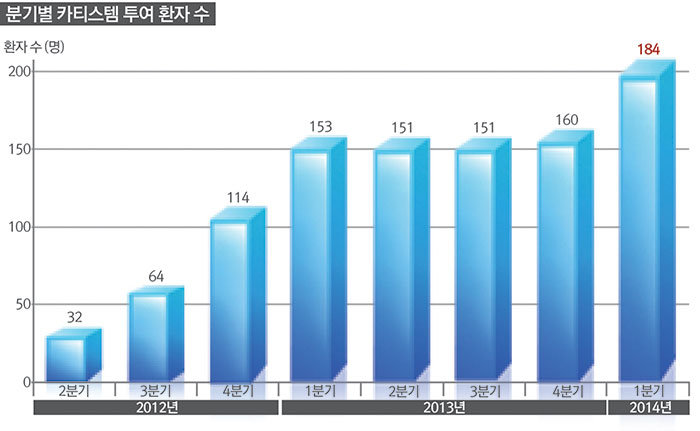
‘CARTISTEM®’ had increasing demand starting from 32 patients in the 2nd quarter of 2012 when the first patient administration occurred, to 64 patients in the 3rd quarter, 114 patients in the 4th quarter and 135 patients in the 1st quarter of 2013.
Thereafter, it showed gradual increase to 151 patients in 2nd quarter of 2013, 151 in the 3rd quarter and 160 in the 4th quarter, but as administration surgery increased again recently, it recorded 184 patients in the 1st quarter of this year.
To this, MEDIPOST stated that “satisfaction of initial ‘CARTISTEM®’ administration patients is very high and preference of medical personnel is also improving in terms of efficacy and safety, therefore administration rate for this ear is anticipated to increase greatly.