
MEDIPOST’s mission is to help patients with incurable diseases and unravel unmet medical needs through stem cells and regenerative medicine.
Since its foundation in 2000, MEDIPOST has been making resilient efforts despite difficulties and challenges in the field of stem cell research and commercialization, while upholding strong corporate ethics and values. Today, MEDIPOST is leading the global stem cell therapeutics field with the world’s first regulatory-approved allogeneic human Umbilical Cord Blood-derived Mesenchymal Stem Cell(hUCB-MSC) product named CARTISTEM® for patients with knee Osteoarthritis(OA), launched in Korean market in 2012.
Biotech industry, including stem cell therapeutics, is deemed to lead the advancement of Korea’s innovative future as the next growth-engine industry. MEDIPOST has been awarded with over 33 public R&D grants for commercialization of stem cell therapeutics in Korea and beyond. MEDIPOST holds a very strong sense of responsibility and pride while striving to overcome the boundaries of currently incurable diseases and unmet medical needs both in Korea and abroad, through our R&D efforts in stem cell technology and commercialization.
MEDIPOST aspires to improve the quality of lives for patients with unmet medical needs through rigorous research, development and commercialization in the field of stem cell therapeutics and regenerative medicine while maintaining strong corporate ethics and values.

CEO and President
Wonil Oh
MEDIPOST’s Vision
Creating a better tomorrow through
Innovative Stem Cell Technology
MEDIPOST aspires to improve the quality of lives for patients with unmet medical needs through rigorous research, development and commercialization in the field of stem cell therapeutics and regenerative medicine.
Company History
Leap to advance into the global cell therapy market.
- Approval of Phase 3 clinical trials for CARTISTEM® (EVA-001) y the MHLW (Ministry of Health, Labour and Welfare), Japan (K&L grade 2~3) (2021)
- Established the subsidiary company ‘IMMUNIQUE’, developing the cord blood-derived immune cell therapy (2021)
- Approval of Phase 2 clinical trials for Injectable drug for knee osteoarthritis ‘SMUP-IA-01’ in Korea (2021)
- Initiated Phase 2 clinical trials for Injectable drug for knee osteoarthritis ‘SMUP-IA-01’ in Korea (2021)
- Initiated Phase 3 clinical trials for CARTISTEM® (EVA-001) in Japan (K&L grade 2~3) (2022)
- Acquired the shares of OmniaBio Inc, CDMO producing Gene-modified Cell therapies in Canada (2022)
- Awarded the ‘Leading Companies in Materials, Parts, and Equipment’ by the Ministry of Trade, Industry and Energy (2023)
- End of Phase 2 clinical trials for Injectable drug for knee osteoarthritis ‘SMUP-IA-01’ in Korea (2024)

Leading the next generation research and development with advanced stem cell platform technology.
- Awarded ‘2016 Frost & Sullivan Korea Excellence Award’ (2016)
- Conducted investigator-initiated clinical trial of PNEUMOSTEM® for the treatment of Intra-Ventricular Hemorrhage(IVH) in premature infants (2016)
- Completed 5-year follow-up of Phase 3 clinical trial patients who received CARTISTEM® treatment (2016)
- Awarded the ‘Excellent Workplace for Women’ award in Gyeonggido Korea (2016)
- Achieved commercial sale of 5,000 cumulative doses of CARTISTEM® for the treatment of knee articular cartilage defects and Osteoarthritis(OA) in Korean market (2017)
- Awarded the ‘Family-Friendly Workplace’ Award by the Ministry of Gender Equality and Family Korea (2017)
- Successfully completed Phase 1/2 (US-FDA) clinical trial of CARTISTEM® for the treatment of patients with knee cartilage defects in the U.S. (2018)
- Achieved commercial sale of 10,000 cumulative doses of CARTISTEM® for the treatment of knee articular cartilage defects and Osteoarthritis(OA) in Korean market (2018)
- Cleared Phase 1/2 clinical trial (Investigational New Drug – IND) approval of NEUROSTEM® by the US-FDA for the treatment of patients with Alzheimer’s Disease(AD) (2018)
- Successfully completed Phase 1/2 (US-FDA) clinical trial of PNEUMOSTEM® for the preventive treatment of Bronchopulmonary Dysplasia(BPD) in the U.S. (2019)
- Cleared Phase 1 clinical trial approval of SMUP-IA-01 in Korea for the intra-articular injection on patients with Osteoarthritis(OA) (2019)
- Received ‘Fast tract Designation’ for PNEUMOSTEM® by US FDA (2019)
- Approval of Phase 2 clinical trial for CARTISTEM® by the MHLW (Ministry of Health, Labour and Welfare), Japan (2019)
- Completed Phase 1 and Phase 2a clinical trials for NEUROSTEM® in Korea (2020)
- Successfully completed Phase 1 clinical trial of SMUP-IA-01 in Korea for the intra-articular injection on patients with Osteoarthritis(OA) (2020)
- Signed an agreement to transfer MLSC technology, a high-efficiency cell culture platform technology with LG Chemical (2020)

Expanding the future of regenerative medicine on to the global stage.
- Invited to present keynote address at the ‘7th World Stem Cell Summit, California USA’ (2011)
- Cleared Phase 1/2 clinical trial (Investigational New Drug – IND) approval of CARTISTEM® by the US-FDA – a first IND using umbilical cord blood-derived stem cells (2011)
- Cleared first-in-human Phase 1 clinical trial approval of NEUROSTEM® in Korea for the treatment of Alzheimer’s Disease (2012)
- Received Biologics License Application(BLA) market-authorization of CARTISTEM® in Korea – world’s first regulatory approval of allogeneic stem cell product, for the treatment of degenerative Osteoarthritis (2012)
- Achieved first export of stem cell product – CARTISTEM® from Korea to Hong Kong (2012)
- Selected as ‘Outstanding Achiever with Government R&D Support’ by the National Research Council of Science & Technology, Korea (2013)
- Cleared Phase 2a clinical trial approval of NEUROSTEM® in Korea for the treatment of patients with Alzheimer’s Disease(AD) (2013)
- PNEUMOSTEM® for the preventive treatment of Bronchopulmonary Dysplasia(BPD) was designated as ‘Orphan Drug’ by the US-FDA (2013)
- Awarded the Grand Prize (MFDS Minister’s Award) at the inaugural ‘Korea New Drug Award’ Korea (2013)
- New Pangyo headquarter was established with CELLTREE® cord blood bank and R&D Center at the Pangyo Techno Valley, Gyeonggi-do Korea (2014)
- Cleared Phase 1/2 clinical trial (Investigational New Drug – IND) approval of PNEUMOSTEM® by the US-FDA for the preventive treatment of Bronchopulmonary Dysplasia(BPD) (2014)
- PNEUMOSTEM® for the preventive treatment of Bronchopulmonary Dysplasia (BPD) was designated as ‘Orphan Drug in development’ by the Ministry of Food & Drug Safety(MFDS) Korea (2014)
- PNEUMOSTEM® for the preventive treatment of Bronchopulmonary Dysplasia(BPD) was designated as ‘Orphan Drug’ by the EMA (2015)
- Achieved 200,000 cumulative units of private cord blood storage at CELLTREE® (2015)
- Established a Joint Venture Company(JVC) Shandong ORLIFE Pharmaceuticals Co., Ltd. in China (2015)
- Established a Joint Venture Company(JVC) EVASTEM Therapeutics Co., Ltd. in Japan (2015)
- Selected as the host organization of the national ‘Global Advanced Biopharmaceutical Technology Development Project’ Korea (2015)
- Launched a cosmetics product range CELLPIUM® containing cord blood stem cell culture medium (2015)

Tackling unmet medical needs with cutting-edge stem cell technologies.
- Appointed as technical advisor for the establishment of Seoul Municipal Public Cord Blood Bank (2006)
- Established Good Manufacturing Practice(GMP) cell manufacturing facility in Seoul (2006)
- Launched nutritional/dietary supplement brand MOVITA® (2007)
- Became the first cord blood bank in Korea to supply public cord blood unit for overseas transplantation (2008)
- Received the Innovation Award in biotechnology sector, at the Asia Innovation Conference (2009)
- Received the nationwide Outstanding Achievement in R&D Award by the Ministry of Trade, Industry and Energy of Korea (2009)
- Cleared first-in-human Phase 1 clinical trial approval of PNEUMOSTEM® for the preventive treatment of Bronchopulmonary Dysplasia(BPD) (2010)
- Successful completion of investigator-initiated clinical trial for the treatment of cerebral palsy using stored autologous cord blood units – a first by any cord blood bank in Korea (2010)

Presented a new paradigm for treating incurable diseases with stem cell technology.
- CELLTREE® cord blood bank was launched for private cord blood banking service (2000)
- R&D Center for commercialization of stem cell therapeutics launched (2001)
- Received the Best Venture Company Award by the Federation of Korean Industries (2002)
- Appointed as a Lead Cord Blood Bank Operator by the Cell Application Project Research Group of the Ministry of Science and Technology, Korea (2003)
- Received the Bronze Tower Award of Industrial Service Merit, Korea (2005)
- Cleared first-in-human Phase 1/2 clinical trial approval of CARTISTEM® for the treatment of knee articular cartilage damage in patients with Osteoarthritis (2005)
- Successful Initial Public Offering(IPO) on KOSDAQ (2005)

Business Overview

Commercialization of allogeneic cord blood-derived stem cell products.
MEDIPOST successfully commercialized allogeneic cord blood-derived mesenchymal stem cell product named CARTISTEM®, as the world’s first regulatory approved allogeneic stem cell product approved by the Ministry of Food and Drug Safety(MFDS) of Korea in January 2012.
CARTISTEM® is the currently only approved cell therapy product for “Treatment of repetitive and/or traumatic cartilage degeneration including degenerative Osteoarthritis(OA) without age limit” and is commercially available in Korea. In addition, Phase 1/2 (US-FDA) clinical trial of CARTISTEM® in the U.S. has been completed successfully in 2018.
PNEUMOSTEM® is currently under Phase 2 clinical trial in Korea for the “preventive treatment of Bronchopulmonary Dysplasia(BPD)”, in the US, IND (Investigational New Drug) was filed with the FDA and Phase 1/2 clinical trial was completed in 2018.
MEDIPOST’s next-generation SMUP platform for stem cell products uses advanced proprietary cell selection and culture methods. Intra-articular injectable cell product (code name SMUP-IA-01) for patients with knee osteoarthritis (OA) is currently in Phase 1 clinical trial in Korea. Additional SMUP platform-based cell product candidates are in development for the treatment of diabetic nephropathy and immune disorders.

CELLTREE® – Korea’s first and largest cord blood bank.
Cord blood can only be collected from the umbilical cord of a newborn baby at the time of birth. Cord blood is an invaluable resource of stem cells, particularly the blood (hematopoietic) stem cells which can easily be transplanted to the baby or the baby’s family member, should he/she develop blood-related cancer or diseases requiring blood (hematopoietic) stem cell (such as bone-marrow) transplantation.
With MEDIPOST’s stem cell technology, know-how and experience, CELLTREE® Cord Blood Bank provides the highest quality stem cell separation, processing, freezing and storage services for this once-in-a-lifetime opportunity to bank valuable stem cells. The cord blood units stored as CELLTREE® Cord Blood Bank have been processed with MEDIPOST’s proprietary protocols (such as Controlled-Rate Freezing methods) ensuring the stem cells from the cord blood are frozen and banked (cryopreserved) in the optimal condition.
CELLTREE® Cord Blood Bank has been recognized for its quality and expertise in stem cell processing and banking technology, and is Korea’s leading cord blood bank with largest market-share and highest number of cord blood units released for transplantation in Korea.

Tailored nutritional and dietary supplement solution, MOVITA®
MOVITA® provides a smart nutritional and dietary supplement solution according to age, gender and lifestyle (such as level of physical activeness or fertility status) for healthier lifecycle management for everyone in your family.
By understanding physical and dietary needs of everyone in the family based on their age and gender, MOVITA® offers core ingredients for best and thorough nutritional supplement needs.
Driven by the physicians (including the founder and CEO) at MEDIPOST, MOVITA® range of products contain safe and effective formulations suitable to care for the Korean/Asian mothers across their pre-, during- and post-pregnancy periods as well as to care for the nutritional needs of their babies.
Corporate Social Responsibility(CSR)
MEDIPOST values its corporate philosophy : Respect for Life
Based on our corporate philosophy to respect the value of human lives, MEDIPOST is engaged in sponsorship and charity activities for funding biotechnology research activities for finding cures for the intractable diseases and supporting families in need.
Cord Blood Banking Sponsorships
- Providing Cord Blood Bank services for multi-child families
- Free Cord Blood Storage for Siblings of Children with Leukemia

Research & Clinical Sponsorships
- Basic research funding at the Seoul National University College of Medicine
- Providing Financial Support for Underprivileged Patients
- Providing free-of-charge Cord Blood Transplantation for Children with Cerebral Palsy

Charity Activities
- Heart-Share Blood Donation Campaign
- Supporting 1,600 Married Couples with Infertility
- Supporting the Children with Leukemia through creativity classes
Global Networks
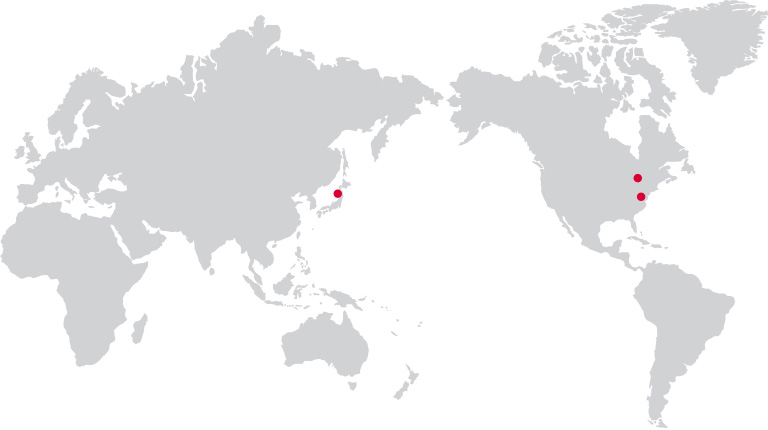
Overseas
· MEDIPOST Inc.
245 Main St. Cambridge, MA 02142, USA
· OmniaBio Inc (CGT CDMO)
175 Longwood Rd. S Suite 201A Hamilton, ON L8P 0A1, Canada
· MEDIPOST K.K.
Masumoto Bldg, 5F, 1-7-6 Toranomon,Minato-ku Tokyo, 105-0001 Japan
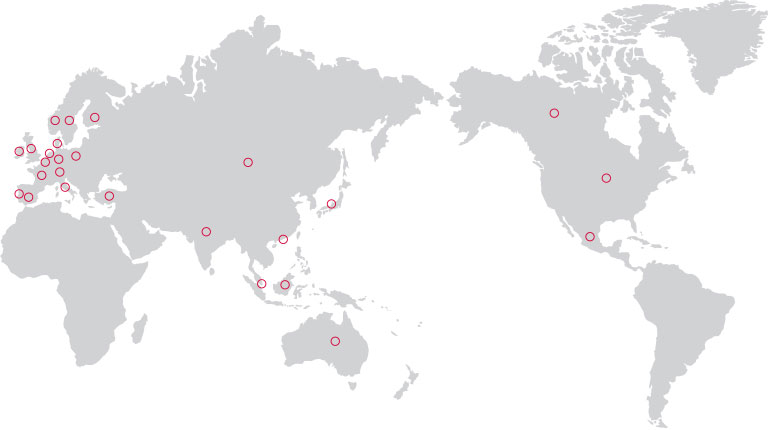
Patent Right
U.S.A. / ENGLAND / SWITZERLAND / SINGAPORE
SPAIN / CHINA / ITALY / CANADA / JAPAN
AUSTRALIA / FRANCE / HONG KONG / SWEDEN
NETHERLANDS / INDIA / MEXICO / GERMANY
AUSTRIA / BELGIUM / DENMARK / FINLAND
IRELAND / NORWAY / POLAND / PORTUGAL
TURKIYE
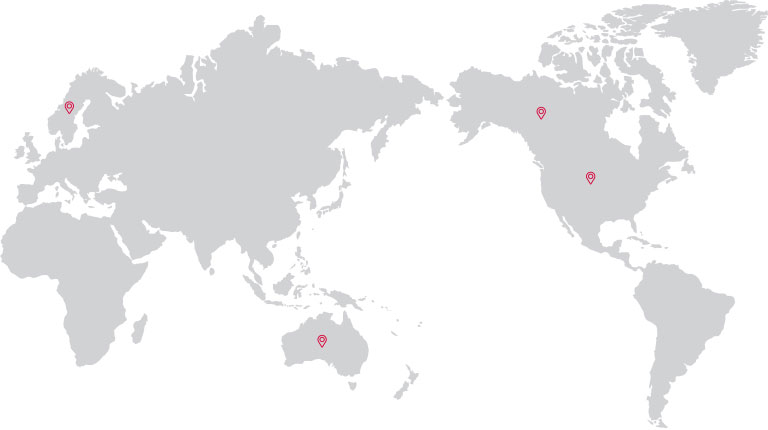
Cord Blood Transplant
· U.S.A.
· CANADA
· SWEDEN
· AUSTRALIA
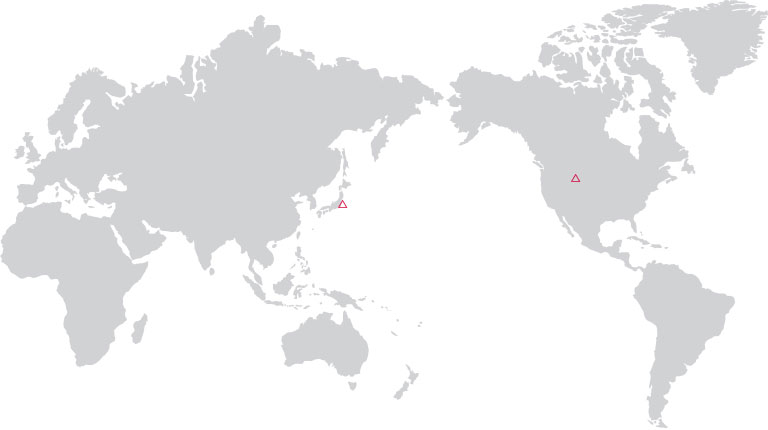
Clinical Trial
· U.S.A.
· JAPAN

